What do we know?
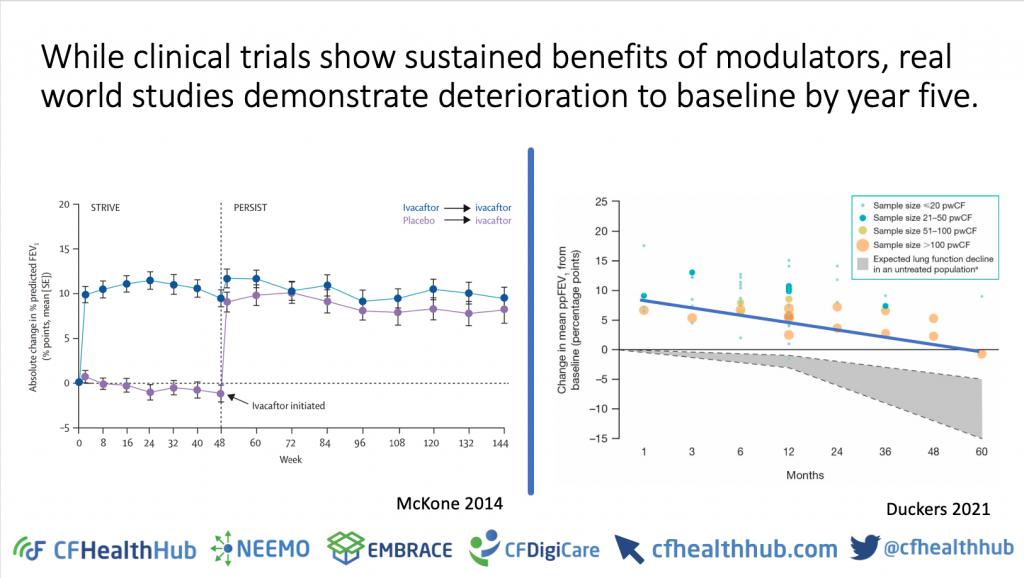
- Ivacaftor efficacy in clinical trials is 9.9-10.6% improvement in FEV11. Effectiveness is around 6%2.
- This efficacy-effectiveness gap is unlikely to be explained by patient characteristics between clinical trial and real-world studies.
- Maintenance therapy continued in the clinical trials1 but there is real world evidence of declining inhaled therapies use following Ivacaftor initiation3,4.
- Body mass index (BMI) continues to improve over the first five years of treatment with Ivacaftor while lung function declines back to baseline by year five, suggesting a lung target to optimise Ivacaftor outcomes5.
- Results of Ivacaftor real-world evaluation were only available six years after the widespread introduction of Ivacaftor in the UK2.
- In the four years since the efficacy-effectiveness gap was identified, the reasons, and therefore potential targets for intervention to close the gap, haven’t been systematically investigated or explained.
- Traditional real-world evaluation of Kaftrio planned by NICE in co-operation with Pharma and the UK CF Registry is likely to take some time.
The importance
- Drug cost which may exceed £100k per patient per year6,7.
- Ivacaftor efficacy-effectiveness gap identified but mechanism still unknown.
- Unlike Ivacaftor, Kaftrio is suitable for the majority of the UK CF population so the need to understand optimal use is greater.
- Kaftrio is highly efficacious; 14.3% between-group FEV1 difference over six months8 but likely that an efficacy-effectiveness gap will also exist and may be exacerbated by emphasis in the CF community around reducing treatment burden by dropping inhaled therapies following the initiation of modulators9,10.
- The data analytic teams at NICE have sign-posted the intention to use digital learning health systems to deliver a real time rapid health technology assessment with the aim of identifying efficacy-effectiveness gaps. The CFHealthHub digital learning health system is well-configured to deliver this.
- There are changes in treatment prescriptions and treatment-taking following modulator instigation and uncertainty for pwCf and clinicians about how to manage this.
The plan
A prospective, observational study using real-time data collected routinely via the CFHealthHub digital learning health system to take an initial look at the efficacy-effectiveness gap for Kaftrio, and the potential impact of adherence to inhaled therapies on the efficacy-effectiveness gap.
This approach also aims to have an intervention component that closes the efficacy-effectiveness gap in a real-world setting by generating new evidence through data analytics using data from a range of sources in real-time8. Find more details here:
Aims
A first look within the first seven months of Kaftrio initiation at the:
- Real-world effectiveness of Kaftrio among adults with CF
- Efficacy-effectiveness gap for Kaftrio among adults with CF
- Change in adherence during inhaled therapies among adults with CF
- Potential impact of objective adherence to inhaled therapies on the efficacy-effectiveness gap
- Potential impact of medication possession ratio (MPR) to Kaftrio on the efficacy-effectiveness gap
Follow us on Twitter: @NEEMOcf

References
1. Ramsey BW, Davies J, McElvaney NG, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. Nov 3 2011;365(18):1663-72. doi:10.1056/NEJMoa1105185
2. Bessonova L, Volkova N, Higgins M, et al. Data from the US and UK cystic fibrosis registries support disease modification by CFTR modulation with ivacaftor. Thorax. Aug 2018;73(8):731-740. doi:10.1136/thoraxjnl-2017-210394
3. Hubert D, Dehillotte C, Munck A, et al. Retrospective observational study of French patients with cystic fibrosis and a Gly551Asp-CFTR mutation after 1 and 2years of treatment with ivacaftor in a real-world setting. J Cyst Fibros. Jan 2018;17(1):89-95. doi:10.1016/j.jcf.2017.07.001
4. Granger E, Davies G, Keogh RH. Treatment patterns in people with cystic fibrosis: have they changed since the introduction of ivacaftor? J Cyst Fibros. Sep 5 2021;doi:10.1016/j.jcf.2021.08.014
5. Duckers J, Lesher B, Thorat T, et al. Real-World Outcomes of Ivacaftor Treatment in People with Cystic Fibrosis: A Systematic Review. J Clin Med. Apr 6 2021;10(7)doi:10.3390/jcm10071527
6. Bush A, Simmonds NJ. Hot off the breath: ‘I’ve a cost for’—the 64 million dollar question. Thorax. 2012;67(5):382-384. doi:10.1136/thoraxjnl-2012-201798
7. Cohen D, Raftery J. Paying twice: questions over high cost of cystic fibrosis drug developed with charitable funding. Bmj. Feb 12 2014;348:g1445. doi:10.1136/bmj.g1445
8. Middleton PG, Mall MA, Dřevínek P, et al. Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N Engl J Med. Nov 7 2019;381(19):1809-1819. doi:10.1056/NEJMoa1908639
9. Davies G, Rowbotham NJ, Smith S, et al. Characterising burden of treatment in cystic fibrosis to identify priority areas for clinical trials. J Cyst Fibros. May 2020;19(3):499-502. doi:10.1016/j.jcf.2019.10.025
10. Gifford AH, Mayer-Hamblett N, Pearson K, Nichols DP. Answering the call to address cystic fibrosis treatment burden in the era of highly effective CFTR modulator therapy. Journal of Cystic Fibrosis. 2020;19(5):762-767. doi:10.1016/j.jcf.2019.11.007
